Transforming Pharmaceutical Innovation Through Advanced Gut Replication for Smarter Drug Development
About GI Bio
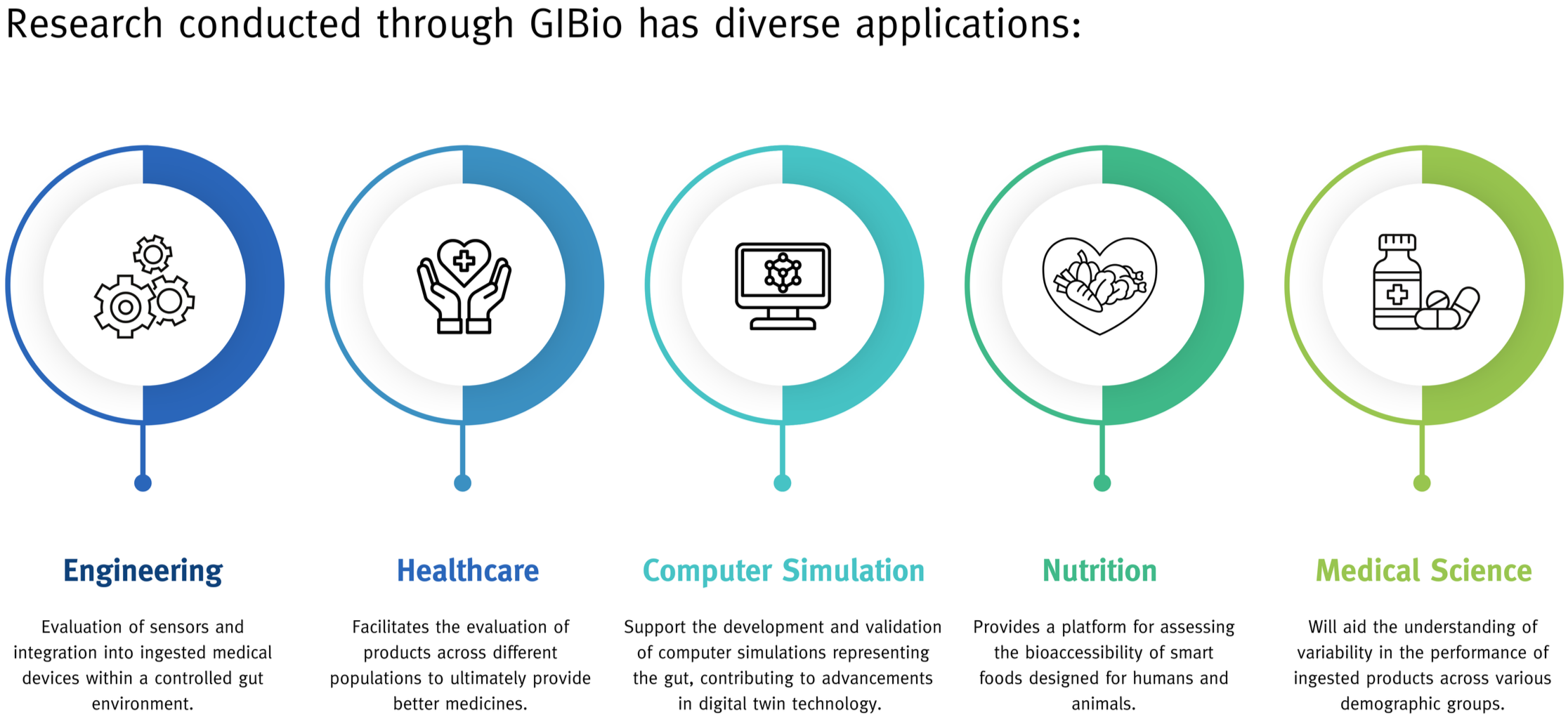
GI Bio addresses a major challenge in understanding the gut's complex dynamic environment by offering a way to replicate it without the limitations of animal or human studies.
This technology facilitates the design, development, and manufacture of ingested products, transforming the process by providing early clinical quality data. As the only accessible facility of its kind in the UK, GIBio is poised to significantly reduce the time and costs associated with creating new drug products.
Oral administration is the primary method for delivering drugs, involving absorption from the gut into the bloodstream via disintegration, dissolution, and transport across the gut wall. These processes are influenced by complex interactions between the gastrointestinal tract, drug, and dosage form, leading to variable delivery performance.
Understanding this complexity is limited, but fine-tuning of the equipment to replicate healthy and diseased gut conditions can lead to the development of patient-centred products, ultimately improving therapeutic outcomes.
Aims
To establish early feedback mechanisms for ingested products by linking product critical quality attributes with in vivo performance, thereby advancing comprehension of how dynamic gastrointestinal conditions shape product efficacy.
To investigate the spectrum of gastrointestinal variability and its impact on in vivo performance, enabling the design of innovative dosage forms tailored to accommodate such variability and promote the development of patient-centric products.
To provide relevant input data for the creation of a digital twin of the gastrointestinal tract, replicating drug dissolution and absorption processes to enhance simulation accuracy and modelling capabilities.
TIM-1
Gastric compartment and three small intestinal compartments: duodenum jejunum ileum
Gastric and intestinal fluid secreted at true-to-life flow rates
Intestinal content is mixed by peristaltic movements
Bioaccessible fraction accessed via hollow fiber-membrane unit
TIM-2
Dynamic in vitro model of the large intestine
System inoculated with dense and highly metabolic active colon microbiota, mimicking in vivo situations
Strict anaerobic conditions maintained to optimally study fermentation and bioconversion of undigested compounds
Fermentation products and other low molecular weight compounds are continuously removed from the lumen via dialysis
TIM-1 and TIM-2 were supplied by InnoGI Technologies (formerly known as The TIM Company).
Alignment with CMAC Strategy:
GIBio's research aligns closely with the CMAC strategy through its integration with the Biorelevant Performance Classification System (BPCS), which aims to aid in the design of optimally performing medicines.
GIBio employs advanced, dynamic dissolution systems, TIM-1 and TIM-2, to evaluate the performance of a drug product. By simulating various gastrointestinal conditions, including diseased states and pediatric populations, and subjecting formulations to them, GIBio feeds back into the optimisation of drug products.
Furthermore, the data generated by TIM experiments can be utilised in physiologically based pharmacokinetic (PBPK) models, enhancing our understanding of a drug's behaviour in the body. Thus, GIBio's activities directly support the overarching goals of the CMAC strategy by advancing pharmaceutical development through comprehensive performance evaluation and optimisation.
The Team
Funders/Partners:
Funded by EPSRC (Engineering and Physical Sciences Research Council). Grant Ref: EP/W036452/1.
External partners include:
Ric Barker – Associate Principal Scientist (AstraZeneca)
Franc Overmars – Chief Operating Officer (InnoGI Technologies)
Susann Bellmann – Chief Technical Officer (InnoGI Technologies)
Paul Blakeman - Strategic Partnerships Manager (CPI)