Solid State Selection Using a Novel Nucleator Coupled With Design of Experiment (DoE)
Academic:
Prof Jan Sefcik (Strathclyde)
Researchers:
Dr Pol MacFhionnghaile (Strathclyde)
Maria Briuglia (Strathclyde)
Thomas Kendall (Strathclyde)
John McGinty (Strathclyde)
Rachel Sheridan (Strathclyde)
Vaclav Svoboda (Strathclyde)
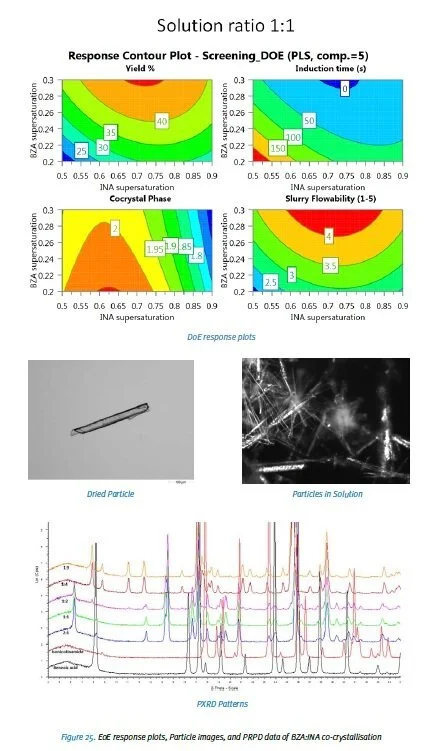
In the production of many materials solid state variation is recognised as a key issue. This is showcased by the strict stance the FDA has taken on polymorphism in manufacturing pharmaceuticals. The Sefcik group have used a Design of Experiments (DoE) based approach instead of a “trial by error” method to produce a selected co-crystal using continuous antisolvent crystallisation. The model molecular complex chosen was the 2:1 benzoic acid: isonicotinamide (BZA:INA) co-crystal1. This is a multicomponent crystallisation in where the INA in solution acts as an antisolvent to the BZA in its solution, and vice versa.
To reduce the complexity of the DoE the known parameters that vary crystallisation were streamlined. By keeping the experiment isothermal and using the tubular nucleator the temperature and mixing were kept constant. The solvent/antisolvent pairing was decided from results of bench screenings. The remaining variables were the solution concentration (saturation), the solvent/antisolvent composition, and the total flow rate.
The first attempt of running the process continuously used 164gkg-1of benzoic acid in ethanol and 48.5gL-1of isonicotimamide in water with each running with a mass flow rate of 50g/min. Although this system did crystallise, the 2:1 co-crystal the crystallisation rate was too great causing fouling and blockage to the tube. Because of this the DoE was revisited to reduce the crystallisation rate. Modified conditions of 110gL-1of benzoic acid in ethanol and 48.5gL-1of isonicotimamide in water were chosen as starting solutions and ran with total mass flow rate of 20g/min each. The system ran for 19 minutes without fouling. Characterisation by Powder X-Ray Diffraction, IR spectroscopy and Raman microscopy confirmed the product to be the 2:1 co-crystal. The particles were characterised as long needles using a Malvern Morphologi.