Peptide Isolation via Spray Drying: Investigating Particle Formation and Means of Process Implementation
Techniques for the isolation of peptide-based systems with highly targeted therapeutic efficacy are of increasing interest for the pharmaceutical industry in order to accelerate product development.
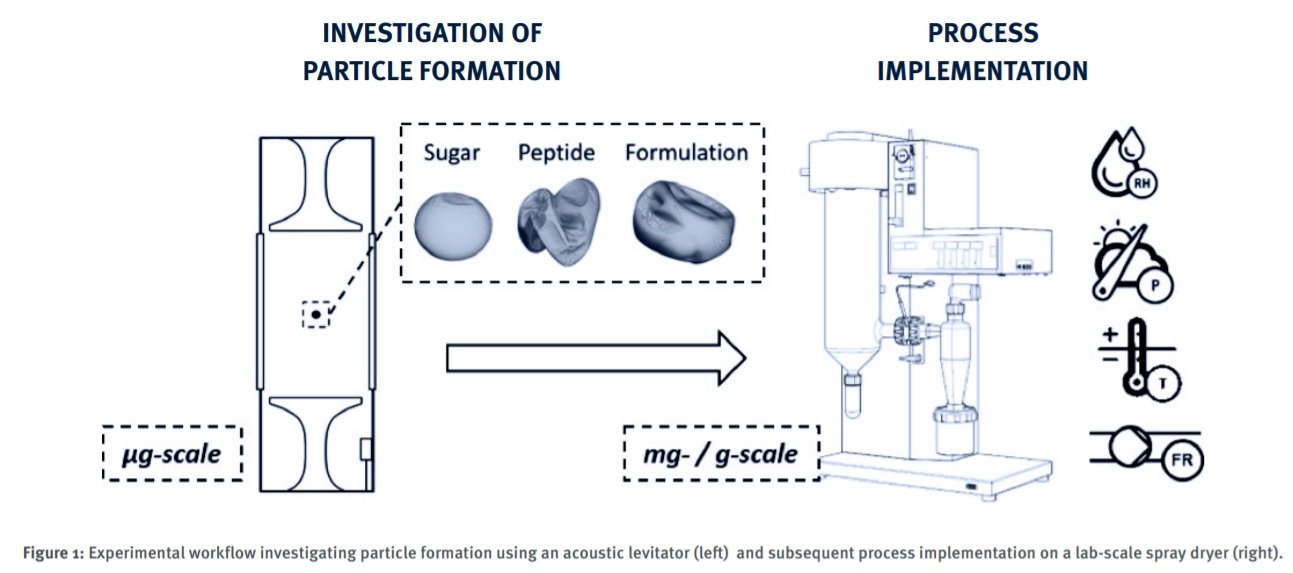
The PhD Industrial Placement between CMAC and Eli Lilly aimed to investigate the feasibility of, and develop a guideline for, the implementation of spray drying as an isolation technique for novel pharmaceutical peptides. The project was a structured experimental investigation on the impact of process parameters and solvent composition on product attributes relevant to manufacturability and drug performance.
““This pre-competitive project was a great success in not only further demonstrating spray-drying techniques for an alternative isolation process for peptides, but also in utilizing and applying new capabilities for data capturing at laboratory scale, which should facilitate more rapid implementation to manufacturing scale. The collaborative nature of the consortium presented fruitful opportunities for the exchange of both techniques and experiences, and the CMAC student exhibited outstanding learning agility in delivering on the project goals.” ”
Highlights:
Single droplet evaporation experiments in an acoustic levitator to investigate particle formation Spray drying platform characterisation to guide process implementation using established and in-house developed
Process Analytical Technology Off-line product characterisation for a broad range of particle properties with a direct impact for downstream processing and product efficacy
Successful identification of optimised process conditions and application for the production of spray dried s-Glucagon as a model peptide system
Project Benefits and Impact
The project demonstrated the potential of using spray drying as an alternative to currently employed lyophilization isolation techniques. This could potentially reduce the overall cycle time by up to 40% for the drug product process.
Introducing capabilities for data capturing to monitor future lab-scale experiments and support process implementation for novel (bio-) pharmaceutical systems.
Extending the existing workflow of off-line analysis techniques with a new approach for the characterisation of peptide fibrillation. This semi-quantitative assessment will be utilised for product evaluation and early process development.
““The project was an excellent opportunity to translate developed capabilities from my academic research to a pre-competitive industrial project exploring spray drying as a manufacturing strategy for novel pharmaceutical peptides.
The project highly benefitted from a multi-disciplinary team of industrial specialists at Eli Lilly. It was a fantastic personal experience to become part of this innovative team.”